10+ Tarveda Therapeutics
Tiragolumab plus atezolizumab was well tolerated with a safety profile generally. Web Langer has been involved in the founding of numerous biotech and med-tech companies including Semprus Biosciences Frequency Therapeutics Tarveda Therapeutics Microchips Biotech and many more.

Sec Filing Roivant Sciences Ltd
Web Tiragolumab plus atezolizumab showed a clinically meaningful improvement in objective response rate and progression-free survival compared with placebo plus atezolizumab in patients with chemotherapy-naive PD-L1-positive recurrent or metastatic NSCLC.

. Web Antibody-drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. The cell membrane CM protects intracellular components from the surrounding environment. 13 Almost all natural membranes.
He holds over 1300 patents for biotechnologies. Web Some of the prominent NET treatment market companies are BoehringerIngelheim International GmbH AVEO Oncology Novartis AG Tarveda Therapeutics Hutchison MediPharma Limited Pfizer Inc IpsenPharma and Progenics Pharmaceuticals. FREng born August 29 1948 is an American chemical engineer scientist entrepreneur inventor and one of the twelve Institute Professors at the Massachusetts Institute of Technology.
Web 2020年3月赛生药业获得Tarveda的PEN-866在大中华地区的开发和商业化许可今年赛生医药注射用PEN-866临床申请获国家药监局受理PEN-866是赛生药业开发的一款小分子偶联药物SMDC目前正在美国开展针对实体瘤的II期篮式试验. A 33 dose escalation design was used. Web Fromkin most recently served as Chief Executive Officer of Tarveda Therapeutics Inc.
In blood plasma cfDNA typically consists of double-stranded. Unlike chemotherapy ADCs are intended to target and kill tumor cells while sparing healthy cellsAs of 2019 some 56 pharmaceutical companies were developing ADCs. Questions Answered By This Report.
However inefficient in vivo mRNA delivery along with a requirement for immune co-stimulation present major hurdles to achieving anti-tumor therapeutic efficacy. Web Furthermore detectable HPV DNA and the concentration of HPV DNA in saliva following treatment were both associated with a significantly higher risk of disease recurrence HR 107 95 CI 2364850. 03 2022 GLOBE NEWSWIRE -- The Global NET Treatment Market Size accounted for USD 2741 Million in 2021 and is estimated to achieve a market size of USD 6336.
This site tracks which universities and pharmaceutical companies are doing this and which arent. 101200JCO2003555 Journal of Clinical Oncology - published online before print July 22 2021. P 0002 and inferior OS HR for death 259 95 CI 323208.
4 King Abdulaziz University Jeddah 21589 Saudi Arabia. Here we demonstrate a proof-of-concept. ADCs are complex molecules composed of an antibody linked to.
1 4 Epithelial ovarian cancer is the leading cause of death from gynecologic cancer in the United States and is the countrys fifth most common cause of cancer. What was the market size of NET. Fromkin served in various roles for Clinical Data Inc including Executive Vice President October 2005 until May 2006 President Chief Executive Officer and Director May 2006 until May 2011.
Web We also examined the durability of T-cell responses. Web 1 Introduction. Web Synthetic mRNA represents an exciting cancer vaccine technology for the implementation of effective cancer immunotherapy.
Web Robert Samuel Langer Jr. He received the 2007 NSTI Fellow Award and 2012. In phase Ib dose expansion patients had checkpoint.
He was formerly the Germeshausen Professor of Chemical and Biomedical Engineering and maintains activity in the Department of. Web This Review highlights recent progress in precision therapeutics and drug delivery and identifies opportunities for strategies to improve the therapeutic index of cancer drugs and consequently. Web By law all clinical trials on the European Union Clinical Trials Register EUCTR must report their results in the registry within a year of completion.
Formerly Blend Therapeutics Inc. From 2005 until 2011 Mr. Ovarian neoplasms consist of several histopathologic entities with epithelial ovarian cancer accounting for the majority of malignant ovarian neoplasms 90.
Medical writing assistance was provided by Liz Leight PhD of Amgen. In this first randomized phase 3 trial for a KRAS G12C inhibitor oral sotorasib demonstrated superior PFS and ORR compared to intravenous docetaxel and had a more favorable safety profile. Web The term cell-free DNA cfDNA refers to fragments of DNA that are present outside of cells that can be detected within bodily fluids.
101038nrc2016108 Abstract The intrinsic limits of conventional cancer therapies prompted the development and application of various nanotechnologies for more effective and safer. To evaluate AZD4635 an adenosine A2A receptor antagonist as monotherapy or in combination with durvalumab in patients with advanced solid tumorsPatients and Methods. During dose escalation CT900 doses of 16 mgm2 weekly and 212 mgm2 every 2.
We found that CD8 T cells were greatest on day 10 20 following administration of mRNA A18 LNPs on day 1 and day 6 and that cells were. Web The estimated restricted mean survival time for progression-free survival after 24 months of follow-up was 137 months 95 CI 120 to 154 in the pembrolizumab group as compared with 108. Web 3 Tarveda Therapeutics Watertown Massachusetts 02472 USA.
Aymeric is an elected member of the World Economic Forums Young Global Leaders Class of 2013 and the Swiss Academy of Engineering Sciences 2016. More specifically the CM maintains cell homeostasis provides structural support maintains ion concentration gradients and controls the entry and exit of charged small molecules and nutrients. Web He is currently a board member of Crocus Technology H55 Natron Energy Tarveda Therapeutics and Selecta Biosciences.
Web Acumen Research and Consulting recently published report titled NET Treatment Market Analysis Report and Region Forecast 2022 - 2030 LOS ANGELES Nov. CT900 is a novel small molecule thymidylate synthase inhibitor that binds to α-folate receptor α-FR and thus is selectively taken up by α-FRoverexpressing tumorsPatients and Methods. In phase Ia dose escalation patients had relapsedrefractory solid tumors.
P 0002 115 and in a separate study were demonstrated to.

1 3 18 Tarveda Therapeutics Provides Year End Update And Outlines Milestones For 2018 Tarveda Therapeutics
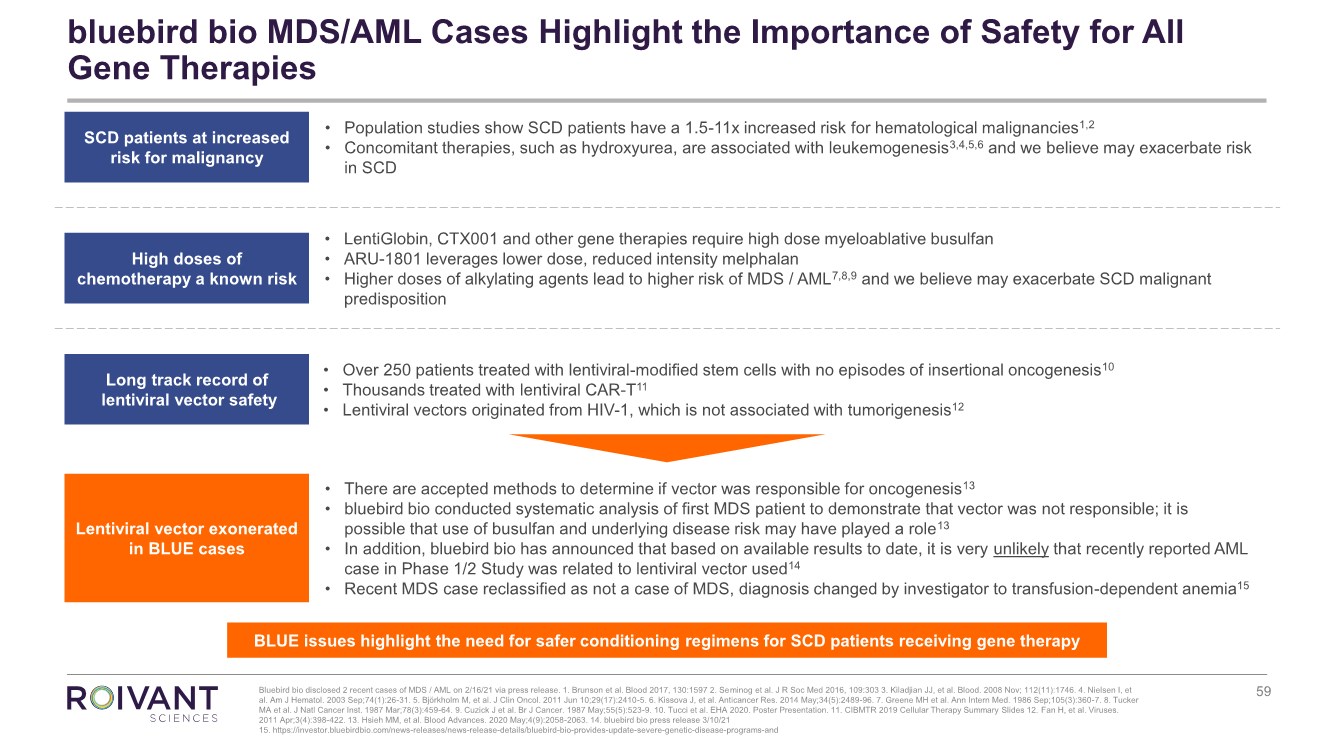
Tm2119232d5 Ex99 1img059 Jpg

Organovo And Tarveda Therapeutics Announce Definitive Merger Agreement Business Wire
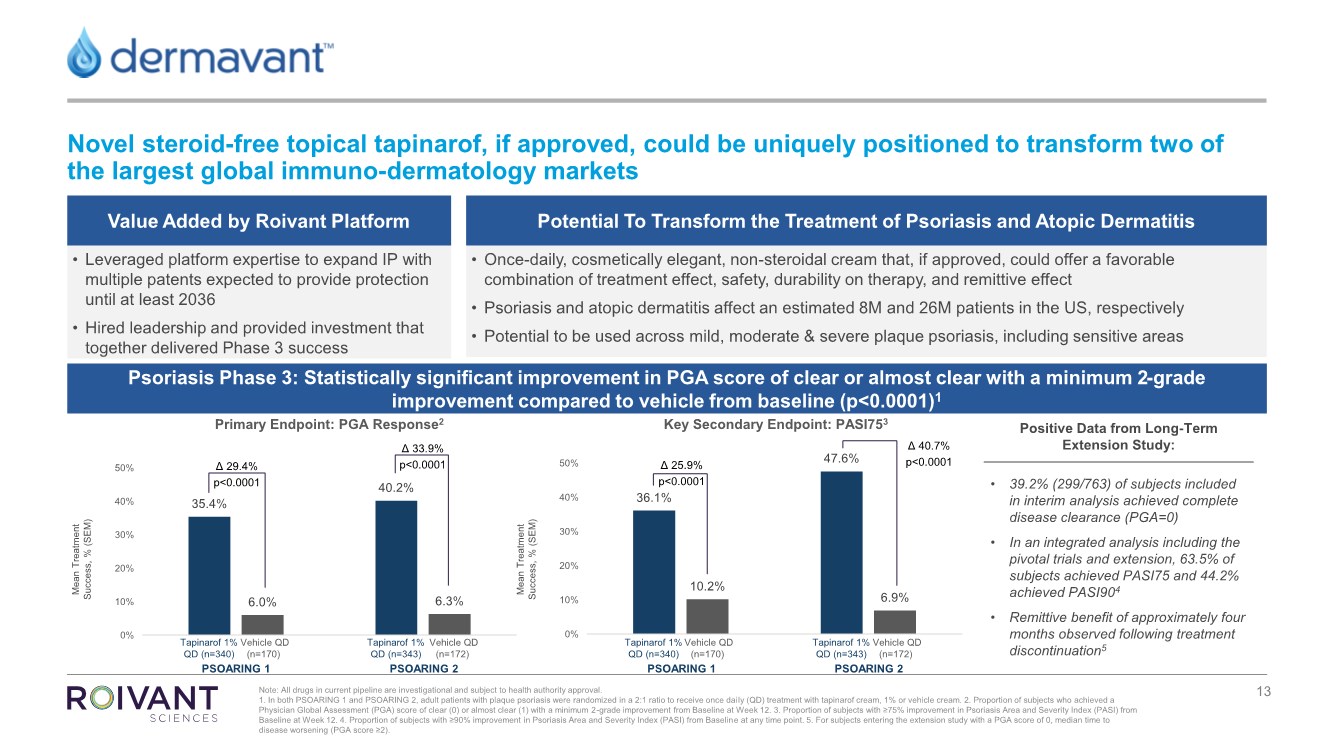
Tm2119232d5 Ex99 1img013 Jpg

10 Rakhade Profiles Linkedin

Ex 99 1
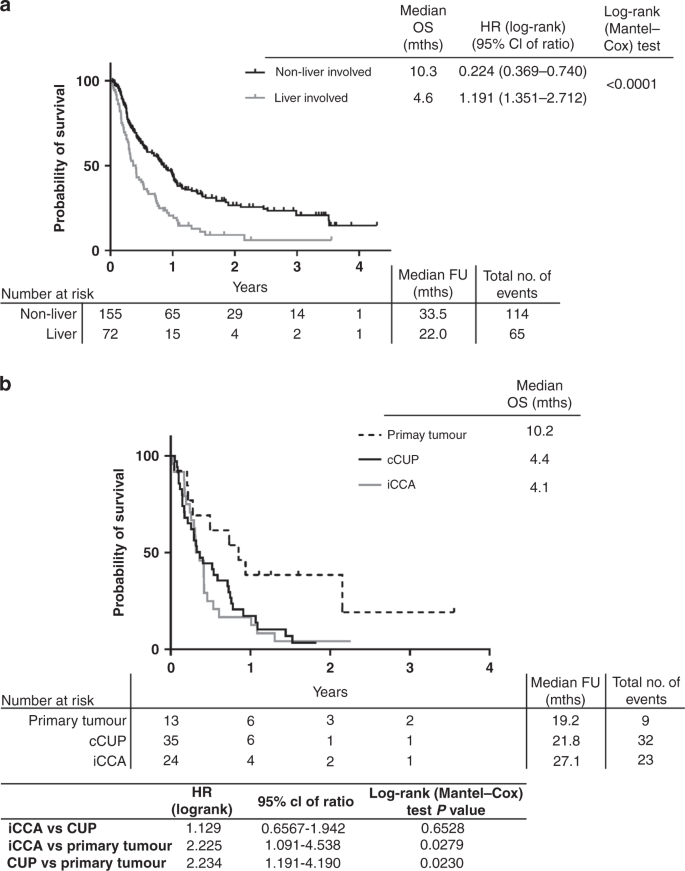
Intrahepatic Cholangiocarcinoma Hidden Within Cancer Of Unknown Primary British Journal Of Cancer

Edward Garmey Consultant Cmo Tagworks Pharmaceuticals Linkedin
Tarveda Therapeutics Company Information Funding Investors Dealroom Co

Pdf Large Scale Characterization Of Drug Responses Of Clinically Relevant Proteins In Cancer Cell Lines

Edward Garmey Consultant Cmo Tagworks Pharmaceuticals Linkedin

Tm2119232d5 Ex99 1img046 Jpg
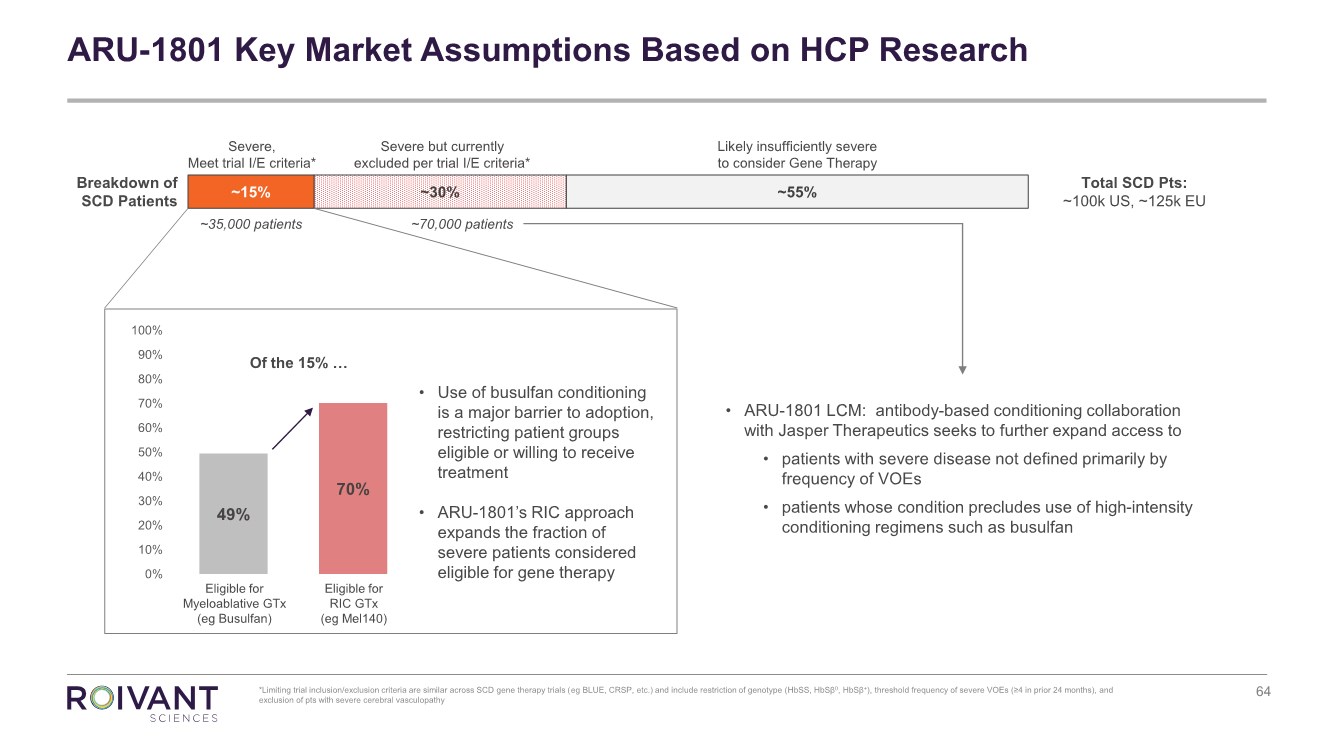
Tm2119232d5 Ex99 1img064 Jpg
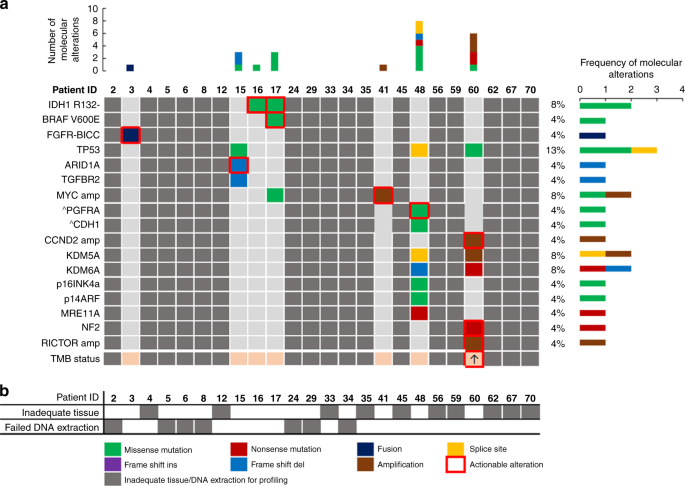
Intrahepatic Cholangiocarcinoma Hidden Within Cancer Of Unknown Primary British Journal Of Cancer

Charles Andre Lemelin Scientific Fellow Process Chemistry Tarveda Therapeutics Linkedin

Marissa Callahan University Of Massachusetts Amherst Boston Massachusetts United States Linkedin

Sec Filing Roivant Sciences Ltd